The Body Electric
by Robert O. Becker
Eleven
The Self-Mending Net
Spinal paralysis is the most devastating of injuries and also one of the
commonest; it afflicts over half a million Americans, including fifteen
thousand new sufferers every year. Until recently their outlook was absolutely bleak, for the human central nervous system (CNS) had no known
regenerative capacity whatsoever. Only if part of the spinal cord remained unsevered was some recovery possible with physical therapy.
Now, however, there's hope that we'll soon be able to coax nerve cells
into reestablishing the proper connections across the damaged section
and thus return the use of arms, legs, sexual and excretory organs, respiratory muscles, and the sense of touch to quadriplegics and paraplegics.
In one way or another, this dream involves making human nerve cells
behave more like those in simpler animals.
The neuron is the basic unit of all nervous systems. It consists of a cell
body, containing the nucleus and metabolic organelles, surrounded by
dozens of filaments that carry messages in and out. The incoming dendrites predominate in sensory neurons. There's usually only one motor
fiber, or axon, which carries the neuron's outgoing messages to dendrites
of other neurons or to receptors on muscle or gland cells. An axon, often
several feet long, is the principal fiber of a motor neuron, which relays
orders from the brain or spinal cord to the tissues and organs.
All neuron cell bodies reside in the brain and spinal cord. Only their
axons and dendrites extend outward, forming the peripheral nerves that
connect every part of the body with the CNS. Other fibers connect certain sensory and motor neurons within the spinal cord, creating reflex
arcs, like those that jerk our hands from hot stows without our having to send the impulse all the way to the brain for instructions. Still other
fibers connect spinal neurons with those in the brain, and in the brain
itself the interconnections reach such a density that each nerve cell may
hook up with as many as twenty-five thousand others.
Except for a few specialized components like the naked fiber tips that
enter into neural epidermal junctions, all parts of every neuron are swaddled in various types of perineural cells. In the brain there are several
kinds, collectively called the glia, in which the neurons are embedded
like hairy raisins in a pudding.
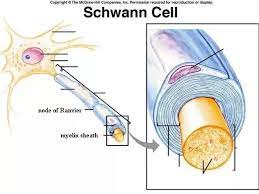
The cell bodies in the cord also are
surrounded by glial cells, but their axons and dendrites, which include the fibers of the peripheral nerves, are surrounded by Schwann cells.
These form tubes, made up of spiraling layers of membrane rich in a
fatty substance called myelin, around some of the largest fibers. A third
type, ependymal cells, line the four cavities within the brain, or ventricles, and the narrow central canal that runs the length of the spinal cord.
These cells are close relatives, having all developed from the same part of
the ectoderm, or outer cell layer, that formed the primitive neural tube
in the embryo. The nervous system actually consists of several times more perineural cells then neurons.
SCHWANN CELL SHEATH AROUND
A PERIPHERAL NERVE FIBER
Until recently the perineural cells were considered merely a "packing
tissue," whose only job was to insulate and support the neurons. We've
now learned that they play a major role in getting nutrients to the neurons. They also help control the diffusion of ions through the nerve cell
membrane and hence regulate the speed of impulse firing, even to the point of inhibiting seizures, the random spread of impulses in the brain.
They may also have an important part in memory, and they probably
conduct the direct currents so important to regeneration. They're essential to healing wherever it occurs in nerve tissue.
Peripheral Nerves
Peripheral nerve fibers can regrow—otherwise we'd lose sensation whenever we cut a finger—but neurons and their fibers in the CNS cannot.
The peripheral nerve's cell body survives, safe in the cord or brain, and
the cut end of the attached part of the fiber is sealed off. The outer,
severed part dies and degenerates; some of its Schwann cells digest it,
along with the now-useless myelinated membrane layers. The empty
Schwann tube remains, however, and begins to grow toward the proximal fiber (the one nearer the center of the body), whose Schwann cells
are also growing across the chasm. When these cells meet, the nerve
fiber grows along its reconnected sheath and eventually makes contact
with the same terminals it originally served.
In salamanders this process is very efficient. The Schwann cells can
cross large gaps, and an experimenter who wishes to work with denervated limbs must be diligent to keep the nerves from reentering. In
humans, the two ends of the tube usually can't find each other over a
distance of more than a centimeter. In that case the proximal sheath
with its intact nerve fiber hunts for its opposite number by growing in
an ever increasing spiral, apparently searching for some signal from the
distal end (the part farther from the body center). Since each nerve is
formed of many fibers, these spiraling tubes entangle each other in a
lump of nerve tissue called a neuroma. Neuromas are painfully sensitive
and often must be cut away. Occasionally a surgeon can move the two
ends of the nerve close enough for the Schwann cells to make contact. If
that's impossible, the gap may be closed with a piece of nerve grafted
from a less important peripheral nerve that can be sacrificed. Unfortunately, nerve grafts don't take reliably, and other methods, such as
making artificial channels with tiny plastic tubules, are still in the experimental stage.
We don't know why the salamander's peripheral nerve regrowth is so
much more effective than ours, but I surmise that its more efficient DC
electrical system accounts for the difference. If the locator signal is electrical, it should be possible to augment it in humans so as to grow nerve
fibers over longer distances. Beginning with a 1974 report from David H. Wilson of the Leeds General Infirmary in England, there have been
some interesting claims that pulsed electromagnetic fields have speeded
recovery of limb function in rats after peripheral nerve damage, but the
effect hasn't yet been substantiated for humans. If these findings hold
up, we may soon be able to boost nerves past their 1-centimeter limit,
even if the action is indirect, and a thorough investigation of the electrical basics could drive nerve regrowth to even greater lengths.
SCHWANN CELLS GUIDE PERIPHERAL NERVE REGROWTH
The Spinal Cord
A sad and crucial difference separates peripheral fibers from those in the
human spinal cord, for the latter don't reconnect over even a fraction of a
centimeter. However, in most injuries relatively few of the neurons
themselves are killed. It's important to realize that most of the cord cells
below the injury don't die. The reflex arcs remain intact. In fact, reflexes
are stronger than normal, because the neurons are now disconnected from the regulating influence of the brain. For the same reason, the
broken bones of paraplegics heal in half the normal time, whereas a bone
will heal very slowly or not at all if its peripheral nerve supply has been
cut. Only the communication between brain and spine is silenced in
paraplegia, and that makes all the difference.
Spinal fibers do reconnect in some animals, notably goldfish and, as
you might expect, salamanders. Their ability seems to decline dramatically with age, however. Jerald Bernstein, a neurophysiologist now at
George Washington University Medical School who has studied goldfish
spinal regeneration extensively, has found that one-year-old fish heal almost all of the damage. This competence declines to about 70 percent at
two years and 50 percent at three. Since salamanders aren't raised in
biological supply houses but rather collected from the wild, any group is
likely to include young and old individuals, making comparisons difficult. In our lab we found that cord regeneration isn't uniform in salamanders, probably due to age differences.
Maturity may reduce the response of the ependymal cells, which are
responsible for the first step. They proliferate outward from the central
canal and bridge the gap in a few days. Marc Singer, in a recent study of
this process, concluded that the ependymal cells extend "arms" radiating
outward, which line up like the spokes of wheels stacked one atop another, forming channels for the regrowing fibers to follow. The nerves
then reestablish their continuity within a few weeks.
SALAMANDER SPINAL-CORD REGENERATION
Bernstein also found that there's a critical period during which regrowth must be completed or it will fail. After cutting the cords of
goldfish, he inserted Teflon spacers to block regeneration. The normal processes took place, but of course the cells couldn't penetrate the divider. After the cellular activity had died down, Bernstein removed the
barriers, but there was no further change. However, when he then cut
off each damaged end, producing an even larger gap and re-injuring the
cord, the cells started from scratch and healed the defect completely.
Thus there's good reason to believe that even long-standing spinal injuries can potentially be regenerated if we can extend the basic capabilities
of human cells.
One would expect to see some healing response in mammals, even if
it fell short. After all, we only need the elongation and reattachment of
fibers, which does take place in peripheral nerves. Instead the opposite
happens. The cord cells die a short distance above and below the injury.
Cysts form near the ends, and, instead of ependyma, scar tissue fills the
gap. Only after this destruction is there an abortive attempt at regrowth. In humans this amounts to only a few millimeters of fiber
elongation many months after the injury. By then it's too late; the ependymal cells and nerve fibers can't penetrate the scar.
CYSTS AND SCARS PREVENT CORD REGROWTH IN MAMMALS
Why the difference between salamanders and mammals? The reason
may lie in the cord's immediate response. In all animals the injury instantly results in spinal shock, during which all neuronal activity is profoundly depressed, especially in the part of the cord still connected to
the brain. Even the simplest reflexes disappear. As the shock wears off,
the cord below the injury becomes hyperactive. Its reflexes become tremendously exaggerated and lead to spastic paralysis of the muscles. The
interesting difference is in the duration of shock. In young salamanders
and goldfish it lasts only a few minutes, but it may endure for over a
week in old ones. In mammals it takes even longer to wear off—as long
as six months in humans.
We made some electrical measurements on salamander and frog spines
in our lab. The injured area turned out to be strongly positive during
spinal shock, even though all direct-current flow ceased in the entire
cord and in the peripheral nerves arising from the part below the
trauma. Then, as the shock resolved, a steadily increasing negative potential appeared, its size reflecting the amount of outgrowth by ependyma and nerve fibers. We found that we'd only rediscovered these
potentials, however. G. N. Sorokhtin and Y. B. Temper had made the
same measurements at the Khabarovsk Medical Institute twenty years
before. The patterns of shock and polarity both correlated, not only with
the cell activity, but with the end result of regenerative success or
failure. A few minutes of shock and a correspondingly short period of
positivity led to full repair of the cord. Longer delays produced incomplete regeneration, and, when the shock and positive potential persisted for five to eight days or longer, the salamander became completely paraplegic.
As far as I know, the only electrical measurements of spinal shock in
mammals were preliminary ones done at our laboratory in conjunction
with Carl Kao of the VA hospital in Washington, D.C. We tested the
severed cord ends in cats for twenty-four hours and found only an increasing positive potential. The situation seemed quite similar to the
electrical difference between salamander and frog limbs. As in most instances, positive potentials appeared to inhibit constructive cellular activity while negative ones fostered it.
An experiment Kao did several years ago provided some supporting
evidence. Kao made two cuts through the spinal cord in each of several
cats, producing a central fragment about 5 millimeters long, separated
from each end. He then grafted pieces of sciatic nerve as spacers in the
two cuts. Typical degeneration with cysts occurred in each end of the
cord but not in the isolated piece. In fact, this part showed some growth
of its ependyma and nerve fibers. The small piece was probably isolated
from the positive potentials produced in the rest of the cord. Hence it
escaped inhibition and grew. It seems the prolonged electrical positivity
of spinal shock is the main roadblock in the way of human cord repair.
It should be possible to cancel that polarity and replace it with a
growth-stimulating negative one, using a properly shaped electrode.
Older injuries in which spinal shock has subsided might require a different input of current, as well as surgical removal of scar and cysts. The
electrode material would have to be chosen carefully, for some metals are
toxic to nerve cells. Also, humans have a low ratio of ependyma compared to spinal neurons, so we might have to add more. However, it
should be relatively easy to culture more ependymal cells from a sample
of the patient's own, and then inject them when we put in the electrode.
Not too many years ago spinal accident victims usually died of infections or other complications quite soon. Now we can prolong their lives,
but only at enormous social, financial, and psychological cost. Looking
ahead, as in the case of heart damage, we now have hope for releasing
regeneration in humans. Actually, the outlook for spinal regrowth is
more promising. The cellular processes are more familiar, and there are a
few groups, like the American Paralysis Association, that sponsor research more imaginatively than the government agencies. Thus the electrical problems in spinal healing may be tackled sooner than in other
fields.
The public imagination has been captured by the computerized muscle-stimulation techniques being developed by Jerrold Petrofsky, an engineer at Wright State University in Dayton. The nationally televised
sight of his patient Nan Davis and other paraplegics taking tentative
steps and pedaling tricycles with their own muscle power was tremendously exciting. But if we can get the body to do the same things by
itself, that will be even better. Any amount of regeneration would only
make other techniques more effective. Even restoring 10 percent of lost
function would be an unimaginable blessing to those who are now helpless. I feel the electrical manipulation of spinal shock must be tested
vigorously now, for this is perhaps the one area where the barriers of
tragedy are closest to being broken.
The Brain
It might seem foolish to expect any regeneration in the most complex of
all biological structures, the brain, yet salamanders, some fish, and most
frogs in the tadpole stage can replace large parts of it, including the
optic lobes and the olfactory lobes, or forebrain, the part from which our
prized cerebral hemispheres developed in the course of evolution. Replacement depends on ingrowth of remaining sensory nerves, the olfactory nerves in the case of the forebrain and the optic nerves for the optic
lobes. When these nerves grow back into the area where brain has been
destroyed, they stimulate the ependymal cells in the brain ventricles,
which proliferate outward into the damaged part and then differentiate
into new neurons and glial cells. If the animal's nose or eyes are removed
so that the injury zone receives no nerve input, no regeneration occurs.
Thus brain regrowth begins much like that of limbs, with the connection of nerve fibers to an epithelial tissue. The ependyma, remember, is
embryologically a close relative of the epidermis, and in fact can be
considered the central nervous system's "inner skin." Since the electrical
environment produced by the neuroepidermal junction is what stimulates cells to dedifferentiate and divide in the salamander limb stump,
and since we started limb regeneration in the rat by crudely mimicking
this signal, it seems likely that a similar stratagem could induce brain
regeneration in animals normally lacking this ability.
A form of shock, called the spreading depression of Leao after its
discoverer, neurologist A. A. P. Leao, occurs after brain injuries. Starting at the site of damage, it extends in all directions until the entire
cortex becomes electrically positive and all its neurons shut down. Leao
studied it only in response to small injuries, when it persists for a few
hours. We don't know whether it occurs in the salamander or how long
it lasts after major damage to the mammalian brain. Concerted study of
Leao's depression combined with experiments in electrically stimulating
the ependymal cells could open the way to self-repair of the human brain.
Recovery from stroke and head wounds taught us long ago that the
brain has a great deal of plasticity; that is, it can reorganize so that
undamaged regions take over tasks formerly done by the lost cells. Supplementation of this ability with even a small amount of regeneration
might make recovery nearly complete for many brain-damaged people.
For the first time in history, neurologists can hope to progress from
describing the brain and cord to mending them. As Geoffrey Raisman of
London's Laboratory of Neurobiology recently reminded his colleagues:
". . . no immutable natural laws have been discovered that forever rule
out repair of the nervous system."
Twelve
Righting a Wrong
Turn
Good and evil often sprout from the same tree, in the body as in Eden.
Nothing illustrates this paradox better than cancer. Today, because of
breakthroughs in genetics, thousands of scientists are searching for oncogenes, bits of DNA that are presumed to pull the trigger that fires the
malignant bullet. It has been known for a long time, of course, that
cancer isn't inherited through egg and sperm the way hemophilia is.
However, many have postulated that the immediate cause of cancer may
be genetic changes in somatic cells. Normally suppressed genes held in
an unnoticed corner of our genetic bookshelves since long ago in our
evolution might be dusted off only when other bodily conditions are
"just wrong." While the premise of this idea is apparently true, biologists have recently concluded that the difference between a normal gene
producing a normal protein and one that could theoretically cause cancer
is a single "typographical error" in a whole chapter of amino acid sequences. Such mistakes happen so often that we would all be riddled
with cancer from infancy if that were all it took to start the disease.
Something else must go awry before a few misspellings can turn the
whole library into gibberish.
Three basic criteria by which a doctor diagnoses cancer must serve as
the starting point in solving the mystery of its cause. First of all, the
disease always always arises not from an alien germ but from a formerly normal
cell of the host's body, and the cancer cells are more primitive than their
healthy precursors. Moreover, this atavism reflects the seriousness of the disease: The simpler the cells, the faster they grow and the harder they
are to treat, whereas a tumor that still resembles its tissue of origin is
less malignant.
The second criterion is growth rate. Cancer cells multiply wildly, in
contrast to the slow, carefully controlled mitosis of normal cells. Going
hand in hand with this uncontrolled proliferation is a similar lack of
control in the structural arrangement of the cells. Their membranes
don't line up in the normal, specific ways, and they form a jumbled
mass instead of useful architecture. As a further result of runaway multiplication, cancer doesn't observe the "boundary laws" of normal tissue.
Instead it encroaches imperialistically upon its neighbors. In addition,
since the cells don't adhere in any kind of structure, some of them are
constantly breaking off, flowing through the blood and lymph, and setting up colonies—metastases—throughout the body.
The third basic criterion of cancer is metabolic priority. The diseased
tissue greedily takes first choice of all nutrients circulating in the blood;
the healthy part of the body gets what's left over. As the tumors disseminate and grow, they consume all available food, and the host wastes
away and dies.
We can make one crucial observation at this point: Except for the lack
of control, all three characteristics—cell simplicity, mitotic speed, and
metabolic priority—are hallmarks of two normal conditions, embryonic
growth and regeneration.
When considering the similarities between an embryo and a tumor,
it's important to keep in mind one difference. Even though contained
within the body of its mother, the embryo is a complete organism, and
the controls over its cells are primarily its own, not those of an adult.
Over thirty years ago in Switzerland, G. Andres probed this relationship
by implanting frog embryos in various body tissues of adult frogs.
Whenever the host didn't simply reject the graft, the embryo degenerated into a highly malignant metastasizing tumor. As a result, Andres
proposed a theory of cancer that remains provocative today: A normal
cell becomes cancerous by dedifferentiation. This change is not dangerous per se, according to Andres, but, because it occurs in a postfetal
animal, the controls that would normally hold these neo-embryonic cells
in check aren't working.
Cancer's relationship to regeneration is even more interesting. In the
latter, a rapid growth of primitive cells having metabolic priority occurs
in an adult, but with proper control as in an embryo Those animals that
regenerate best are least susceptible to cancer. In general, as complexity
increases up the evolutionary ladder to humans, regeneration decreases and cancer becomes more common. Although salamanders
stand about midway in degree of complexity, they're perhaps the least
specialized of all land vertebrates. They have tremendous regenerative
abilities and almost no cancer. Even to give them tumors in the laboratory requires much effort. Adult frogs, on the other hand, have bodies
that are much more specialized for their amphibious way of life; they
regenerate very little and are subject to several kinds of cancer.
In 1948 Meryl Rose decided to see whether the environment of a
salamander's regenerating limb could control the primitive cells of cancer as well as those of the blastema. He took pieces of a type of kidney
tumor common in frogs and transplanted them to the limbs of salamanders. These tumors took better than most, and soon killed the animals
when allowed to spread unchecked. However, when Rose amputated the
leg just below or through the malignancy, normal regeneration followed, and the cancer cells dedifferentiated more fully as the blastema
formed. Then as the new leg grew, the former frog tumor cells differentiated along with the blastema. The frog cells were easily distinguished from salamander cells by their smaller nuclei, and microscopic
study showed frog muscle mixed in with salamander muscle, frog cartilage cells amid salamander cartilage, and so on.
This monumentally important experiment proved Rose's hypothesis
that regeneration's guidance system could control cancer, too. It implied that cancer cells weren't special but merely embryonic cells in a postembryonic body. Rose's work led directly to Andres's theory a few years later.
Unfortunately, biology was still firmly gripped by anti-dedifferentiationism, and these ideas were partly ridiculed, partly ignored. The reaction held back cancer research for decades, because the dogma implied
that carcinogenesis, like differentiation, was irreversible—once a cancer
cell, always a cancer cell. As long as this view was sacred, the only
possible way to cure cancer was to cut it out or kill it with drugs and X
rays. We've been beating that dead horse for fifty years now with tragically modest increases in survival rates. Surgery works only against tumors that haven't yet spread. Chemotherapy depends on differences
between malignant and healthy cells. However, the differences aren't as
great as we would like, because cancer arises, not in some distant
swamp, but from a slight change in our own cells.
Therefore chemotherapy and X rays inevitably produce some damage in normal cells, too.
Doctors, being only human and sharing in their patients' pain and terror, have devised ever more ferocious treatments. In our war on cancer
we've stampeded ourselves into a sort of Vietnam syndrome: To destroy
our foes, we're killing our friends. As Walt Kelly's cartoon character
Pogo observed in that context, "We have met the enemy and he is us."
A Reintegrative Approach
But surely, you may be thinking, if this theory of cancer had any potential for a cure, the research establishment would have considered it. And
surely there would be some supporting evidence. Unfortunately, even
though the detooling and retooling of cells have now been accepted by
all of biology, the old habits still persist throughout most of the granting hierarchy. A few years ago, for example, I met a young research
fellow at the National Cancer Institute who wanted to study the regeneration-cancer link. He even showed me his proposal, an excellent
one. I told him he was asking for trouble if he submitted it to NCI, but
he said his boss approved and he was sure he'd get the grant. A month
later he was forced out of the institute, and the project has never been
funded. Nevertheless, supporting evidence, from research on the periphery, does exist.
Since regeneration can't occur without the stimulus and control of
nerves, one would expect them to exert some controlling effect on cancer. They apparently do. As far back as the 1920s, several experimenters
implanted tumors into denervated areas. Without exception the cancer
cells took root better and grew faster than where the nerves were intact.
The early work on this point was criticized on the grounds that denervation might have reduced the efficiency of the circulatory system, which
in turn would have enhanced malignant growth. Then in the mid-1950s
and 1960s more sophisticated techniques established the same relationship. Absence of nerves accelerated tumor growth, and variations in
the blood supply had no significant effect.
Further evidence confirming Rose's conclusion that regenerative controls caused tumors to regress came from a series of experiments by F.
Seilern-Aspang and K. Kratochwil of the Austrian Cancer Research Institute in 1962 and 1963. They worked on salamanders, but instead of
implanting frog tumor cells they induced skin cancer with large, repeated applications of carcinogenic chemicals. With persistence they
eventually got tumors that would invade subsurface tissues, metastasize,
and kill the animals. In one series they applied the carcinogen to the
base of the tail; the primary tumor formed there, and metastases appeared at random in the rest of the body. If they then amputated the
tail, leaving the primary tumor intact, this malignancy would disappear
as the tail regrew. Cell studies showed that it didn't die or degenerate
but apparently reverted to normal skin. Furthermore, all the secondary
tumors vanished, too, as though they were being operated by remote
control from the main one. The salamander ended up with a new tail
and no cancer. However, if the primary tumor was at a distant point on
the body, amputation of the tail had no effect. Even though the tail
regenerated, the main cancer and its offshoots all progressed, and the
animal died.
This research, combined with Rose's, indicates that regeneration near
a primary tumor can make it regress by reverting to its normal tissue
type. I doubt that there's anything special about legs or tails; I would predict that regrowth in any part of the body, as long as it was near the
primary tumor, would have the same effect. The key to regression appears to be a change in the malignancy's immediate neighborhood. The
electrical currents in nerve and particularly in the neuro epidermal junction seem likely candidates, since they suffice to start regeneration in
animals normally incapable of it.
There's abundant evidence that the state of the entire nervous system
can affect cancer. Back in 1927 Elida Evans, a student of Carl Jung,
documented a link between depression and cancer in a study almost
totally neglected in the intervening years. In a long-term project begun
in 1946 by Dr. Caroline Bedell Thomas at Johns Hopkins School of
Medicine, students were given personality tests, and the occurrence of
disease among them was charted over several decades. In this and later
studies, a high risk of developing cancer has been correlated with a specific psychological profile that includes a poor relationship with parents,
self-pity, self-deprecation, passivity, a compulsive need to please, and
above all an inability to rise from depression after some traumatic event
such as the death of a loved one or loss of a job. In such a person, cancer
typically follows the loss in a year or two.
Several physicians have found they can greatly increase cancer patients'
chances of a cure with biofeedback, meditation, hypnosis, or visualization techniques. Several years ago O. Carl Simonton, an oncologist, and Stephanie Matthews-Simonton, a psychologist, began using all these
methods, with emphasis on having patients develop a clear picture of the
cancer and their body's response to it. For example, a patient might
spend a meditative period each day imagining white blood cells as
knights on white horses defeating an army of black-caped marauders.
When the Simontons tabulated their first results in "terminal" cases,
they found that, of 159 people expected to die in less than a year, those
who eventually did succumb lived twice as long. The cancer had completely regressed in 22 percent and was receding in an additional 19
percent. These results have held up, and visualization is now being
adopted in some other cancer-treatment programs.
Penn State psychologist Howard Hall, testing hypnosis for boosting
white blood cell activity, found a 40 percent increase in cell counts
among his younger, more responsive subjects just one week after the
trance session. New Haven surgeon Bernie Siegel has developed the Simontons' methods further with therapy groups called Exceptional Cancer
Patients. Having patients make drawings to reveal their true psychological state sans defenses, then working toward a comprehensive change in
outlook (total CNS response) to mobilize the will to live, Siegel has helped his patients greatly improve the quality and quantity of their
lives, as compared to clinical prognoses. This approach also enhances
chemotherapy's effectiveness while minimizing its side effects, and it
dramatically increases the likelihood of a "miracle"—total regression and
cure of the cancer.
All this really shouldn't be so surprising. Under hypnosis the mind
can completely block pain, and research described in the next chapter
has shown that it does so by changing electrical potentials in the body.
How can we be sure it couldn't create appropriate electrical changes
around a tumor and melt it away? There are still major problems with
these psychological approaches, however. Only a minority of people are
able or willing to muster the high level of dedication needed to make
them work, even under the gun of death. Moreover, they require time,
the very thing a cancer patient is short of, and they often don't produce
a complete cure even when practiced diligently. Still, they're encouraging signs that the tide is beginning to turn from the warfare mode to the
simpler—more elegant, as a mathematician would say—ideal of changing the cellular environment that allows a tumor to flourish.
Could we really do the job more directly by applying the proper electrical input to aim a surefire regenerative influence at the tumor? I'm sad
to say that most of the few researchers who've tried electricity against
cancer have used the "kill the enemy" approach. Tumors are somewhat
more sensitive to heat than normal tissue, so some doctors are using
directed beams of microwaves to cook them without, it's hoped, destroying too many healthy cells. FDA approval for general use of this method
is expected soon. It has been known since the time of Burr and Lund
that growing tissue is electrically negative, and cancer is the most negative of all. Hence some researchers have tried to inhibit tumor growth
by canceling the offending polarity with positive current. Early reports
were encouraging, but we now know that toxic metallic ions are released
from most positive electrodes, so this method must be tested with great
caution.
Only one research team has sought a reintegrative effect of electric
current. In the late 1950s Carroll E. Humphrey and E. H. Seal of the
Applied Physics Laboratory at Johns Hopkins tried pulsed direct currents on standardized fast-growing skin tumors in mice. Even though
they used both positive and negative polarities, their results seemed sensational. In one series they got total remission in 60 percent of the test
animals after only three weeks; all the control mice had died by then. In
another series the control tumors averaged seven times the size of the
ones treated with current. Unfortunately, present evidence doesn't really support this approach either. In my group's experiments with human
fibrosarcoma cells in vitro, negative and positive currents both speeded up
growth by over 300 percent. On the other hand, as mentioned in Chapter 8, we found that we could suspend mitosis in the fibrosarcoma cells
with silver ions injected by minute levels of positive current. During one
day of exposure, the cells appeared to dedifferentiate completely, and
they stopped dividing for a month without additional treatment, even
though we changed the nutrient medium regularly. Obviously, this entire subject needs to be investigated more thoroughly.
Some researchers believe pulsed electromagnetic fields may be of some
benefit in treating cancer. Art Pilla, working with Larry Norton and
Laurie Tansman of New York's Mount Sinai School of Medicine, as well
as William Riegelson of the Medical College of Virginia, claims to have
found a pulse sequence that significantly increases the survival time of
mice with cancer. So far, these experimenters say they've increased the
effectiveness of chemotherapy in lab animals with PEMF, but haven't
found a pulse pattern that consistently regresses tumors in vivo, although
Pilla and Steve Smith have been able to transform malignant lymphoma
(lymph node cancer) cells into benign fibroblasts in culture.
The claim that PEMF may retard cancer in animals is seriously
flawed, however. In these experiments the entire animal was exposed to
the field, not just the part with the cancer. The pulsing field (like almost any time-varying magnetic field) induces a stress response in the
animal (see Chapter 15). For a short while this increases the activity of
the immune system, which slows the growth of the tumor. However,
the field's effect on the tumor itself is to speed it up, and, in the long
run, added stress is the last thing an animal with cancer needs. These
experiments cannot be used to indicate the safety or benefit of PEMF in
regard to cancer. Since to heal bones the fields are directed only at small
regions, PEMF as used on humans does not produce stress, increased
immune system response, or any concomitant antitumor activity.
Certain leads, such as the electrically injected silver, remain promising. In the 1950s and 1960s, Dr. Kenneth MacLean published some
interesting work on the use of magnetic fields versus tumors in mice. He
believed he'd healed several cases of cancer with steady-state magnetic
fields, and certain unorthodox healers in America and India who use
permanent magnets have made similar claims. The difference in effect
between steady-state and time-varying fields (see Chapters 14 and 15)
leads me to theorize that a steady-state magnetic field, if strong enough,
may indeed halt mitosis in malignant cells.
Due to the prevailing outlook in cancer research, the key work remains to be done. Even promising non electromagnetic approaches have
been victims of bias. There's sound evidence, for example, that megadoses of vitamin C do slow tumor growth and increase the chances for a
complete cure, but Linus Pauling hasn't been able to persuade any of the
powerful institutes to perform a large-scale trial. Some animal tests are
now being funded, but, since vitamin C is nontoxic, immediate clinical
experiments on large numbers of humans would make much more sense.
In following up the regeneration connection, two experiments in particular are crying out to be tried. Someone must attempt to duplicate
the electrical environment of regeneration around tumors in lab animals,
using electrodes. This would involve introducing small negative currents
to thoroughly test the hypothesis that cancer cells are stuck in a state of
incomplete dedifferentiation. The idea would be to dedifferentiate them
the rest of the way and then let normal processes in the body turn them
into healthy mature cells. The same hypothesis should be tested another
way by surgically creating neuro epidermal junctions near the tumors.
Will these experiments be done soon? I wish I knew. The multibillion-dollar cancer research bureaucracy could certainly afford them,
but, although there are a few signs of change, the establishment is stuck
in the near-primitive state of the war mentality. I've maintained for
many years that we won't learn much more about abnormal growth until
we learn more about the normal kind. That approach can lead to cancer
treatments that are truly compatible with our bodies, far safer and more
effective than the simplistic, dangerous ones now in vogue.
next-211s
The Missing Chapter



No comments:
Post a Comment